Alzheimer’s disease, the most common form of dementia, is a degenerative brain condition that progressively impairs memory, thinking and reasoning skills. It is estimated that it affects 10% of New Zealanders over 65 years and 25% of people over 85. Currently, there is no cure. But there is hope: the international field of Alzheimer’s disease research is massive and scientists are investigating a range of potential therapeutic treatments. One of these is the use of a neuroprotective protein known as “sAPPα”, something that Prof. Cliff Abraham and Associate Prof. Stephanie Hughes along with other colleagues from the University of Otago have been working hard on figuring out.
“The thing that’s always captured my imagination,” Cliff says, “is how the nervous system operates in a way that learning can actually occur. And it’s important to think about how our understanding of the normal and basic mechanisms might become impaired in disease conditions.” Alzheimer’s disease, which is characterised by early memory problems, seemed like the natural place to look.
Some years ago, Cliff and his collaborators, including Prof. Warren Tate and Associate Prof. Joanna Williams, developed an interest in a positively acting protein called secreted amyloid precursor protein-alpha, or sAPPα for short. The protein is known for its ability to improve learning and memory, synaptic plasticity, and neuronal survival. It is also known that in Alzheimer’s disease, sAPPα production is reduced while the toxic amyloid-beta peptide increasingly accumulates in the brain.
Even though the therapeutic potential of sAPPα has been promising, there has been no easy way of delivering it to an animal over a long period of time. The protein normally has to be injected directly into the brain, as it cannot cross the blood-brain barrier (which protects the brain from all kinds of “nasty things” in the bloodstream). However, repeated injections into the brain are just not very practical.
But the arrival of Associate Prof. Stephanie Hughes at the University of Otago opened up new possibilities. In the USA, Stephanie had developed techniques to use viral vectors for gene therapy and brought them back to New Zealand. “Instead of having to inject the protein every day,” Stephanie explains, “we can inject a virus, which delivers the gene that allows expression of that protein by nerve cells. It actually integrates that bit of genetic material into the cells. So it’s a permanent delivery system, a long-term therapeutic option.” And it was exactly what Cliff and his team had been looking for.
They were finally at a point where they could test the therapeutic potential of sAPPα in an animal disease model. Valerie Tan, a PhD student supervised by Stephanie and Cliff, conducted a study to see if they could get long-term expression of sAPPα in the brain to ameliorate the deficits seen in a mouse model of Alzheimer’s disease. They used a one-off injection of a lentiviral vector, a method by which genes can be inserted or modified, and supported by the BRNZ funded Mārama viral vector platform, to express human sAPPα in the nerve cells of a brain area called the hippocampus. The mice, including control groups, went through a series of behavioural tests, some of which showed that sAPPα indeed had therapeutic effects. The treatment with sAPPα prevented deficits in spatial memory tasks – for example, the animals treated with the protein performed better in a water maze test than the untreated animals.
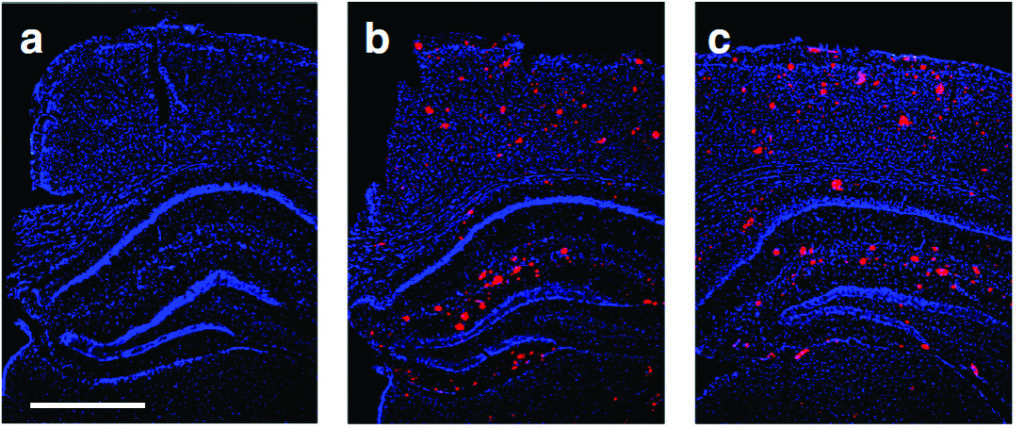

Above: Gene therapy, delivering the therapeutic protein secreted amyloid precursor protein-alpha in a mouse model of Alzheimer’s disease, was found to restore memory function and brain plasticity to normal levels. Interestingly, this occurred without changing the brain pathology, as illustrated in these representative brain sections showing Alzheimer’s pathology (red stains) for a) a normal mouse, b) an Alzheimer’s mouse with gene therapy, and c) an Alzheimer’s mouse without gene therapy. The gene therapy thus enhanced brain resilience against the disease pathology.
When analysing the tissue, the researchers also found that sAPPα affected a synaptic plasticity process called long-term potentiation. “Learning is about changing the connectivity between nerve cells,” Cliff explains, “so that the ones representing a particular experience become more strongly connected, which makes it easier to recall a memory later on. Long-term potentiation is this process of strengthening synaptic connections.” In this work published in Molecular Brain, the researchers showed that the treatment with sAPPα was able to partially rescue long-term potentiation and that deficits could even be fully restored – making it easier to remember events and experiences.
So, what does this all mean for Alzheimer’s patients? Will sAPPα and gene therapy be a viable treatment option? “Gene therapy is certainly coming,” Stephanie says, but there are still some hurdles. The biggest one at the moment is the lack of reliable biomarkers, so that one can predict early on whether someone will get Alzheimer’s disease. Still, gene therapy trials for other therapeutic agents in Alzheimer’s disease are already happening, so a treatment with sAPPα is a possibility.
While our researchers are not putting all their eggs in the sAPPα basket, it certainly is a promising option. “We have found a rescue of brain function without apparently affecting the disease process itself,” Cliff explains. “The hope of sAPPα lies in enhancing cognitive reserve and nerve cell resilience to stave off the effects of the disease for longer.” And indeed, if we could delay the onset of the disease by five years, we could cut down the incidence quite substantially.



