A Brain Research New Zealand funded collaboration – Prof Ruth Empson, Prof Mike Dragunow, Assoc Prof Stephanie Hughes and Dr Andrew Clarkson
PhD student Manju Ganesh (University of Otago) recently won the Sir Grafton Elliot-Smith Poster Award at the 2019 Australasian Neuroscience Society (ANS) conference in Adelaide, Australia. Manju is working on a BRNZ funded project on trialling the transplanting of mouse pericytes into the brain. She works in Prof Ruth Empson’s lab, in collaboration with Prof Mike Dragunow, Assoc Prof Stephanie Hughes and Dr Andrew Clarkson. Here she writes about her project – and shares some amazing images!
Finding new ways to encourage brain self-repair after injury as a realistic and effective therapy for stroke is a major goal of modern neuroscience. A subset of pericytes may hold the key to these efforts as they possess multipotency to trans-differentiate into vascular and neuronal cells in the context of damaged tissue. Pericytes are subtypes of mesenchymal stem cells that ensheath the endothelial cells and reinforce the vasculature.
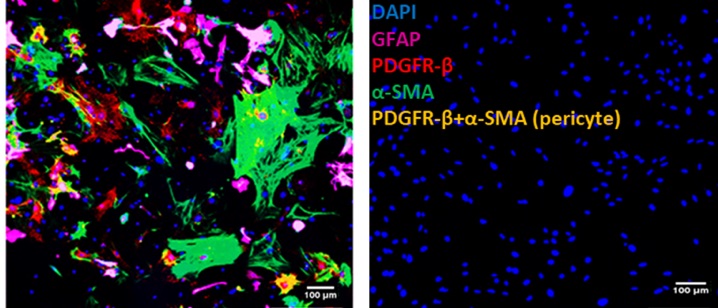
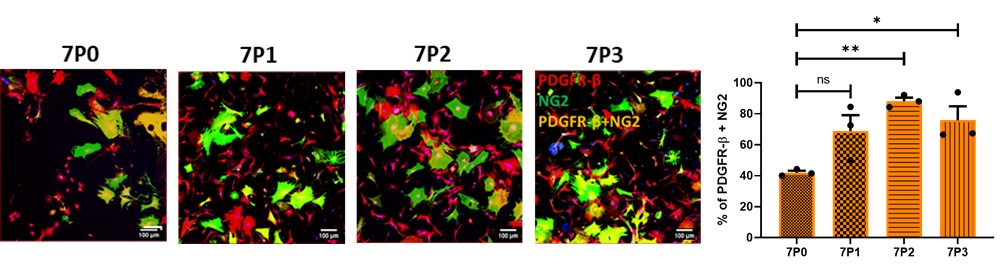
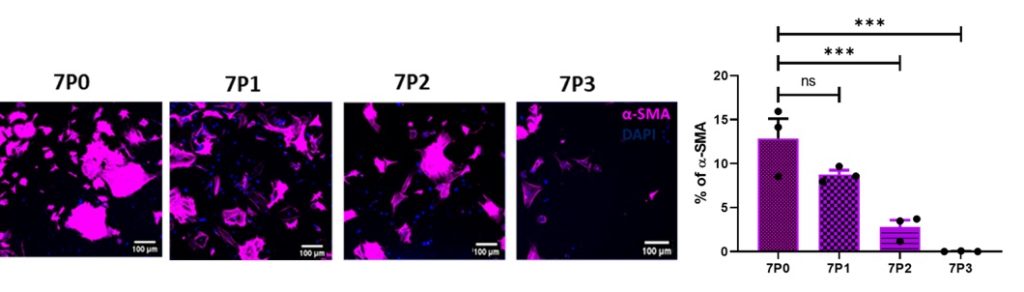
My study aims to understand the role of grafted pericytes within the mouse motor cortex. Mouse brain pericytes were cultured, characterised for the expression of pericyte positive markers (Figure 1) platelet-derived growth factor receptor-Beta (PDGFR-β), neuronal glia (NG2), alpha-smooth muscle actin (α-SMA) and pericyte negative marker glial-fibrillary acidic protein (GFAP). The mixed population was enriched by growing in pericyte specific growth medium (PM) which eliminates α-SMA and enriches for PDGFR-β and NG2 (capillary pericytes) only (Figure 2).
The enriched pericytes were further purified by Fluorescence assisted cell sorting (FACS) using PDGFR-β expression. Pure pericytes were transduced with Lentivirus expressing iRFP (Red Fluorescent Protein) in order to differentiate between the grafted pericytes (24h and 96h post-grafting) and endogenous pericytes and 50,000 cells/µL (iRFP+) were successfully grafted into the mouse motor cortex.
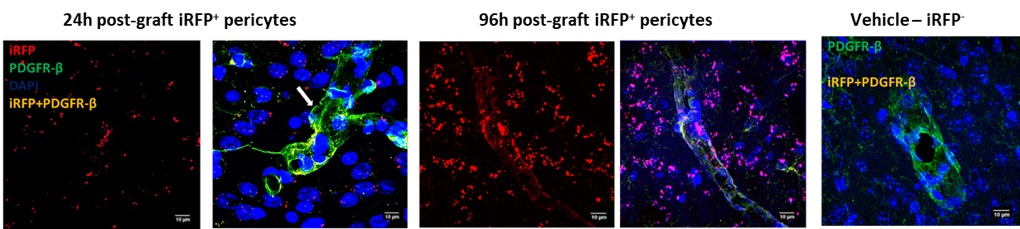
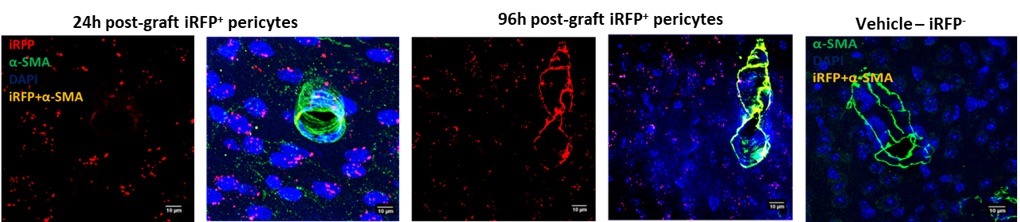
Analysis of the grafted tissues identified the iRFP+ expression amongst pericyte, astrocytic and microglial markers to decipher the fate of grafted iRFP+-pericytes. We observed several grafted iRFP+-PDGFR-β+ and iRFP+-α-SMA+ pericytes along the vasculature (Figure 3). This observation confirms that the grafted iRFP+ pericytes were integrating into the vasculature.
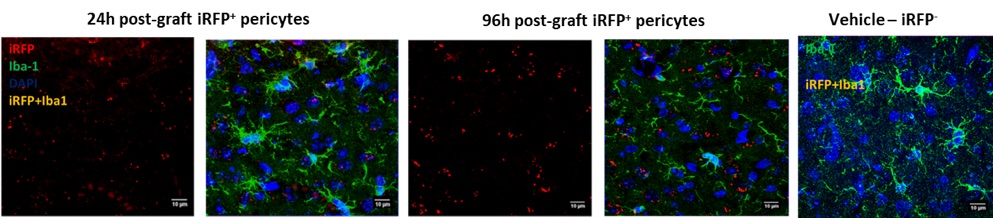
Grafting iRFP+-pericytes also lead to expression of activated microglia (Iba-1) at 24h and it was interesting to observe the reduction in the activated microglia and presence of ramified (healthy) microglia surrounding the graft area by 96h (Figure 4). There was no colabeling of grafted iRFP+-Iba-1+ observed which confirms that the grafted pericytes were not differentiating into microglia.
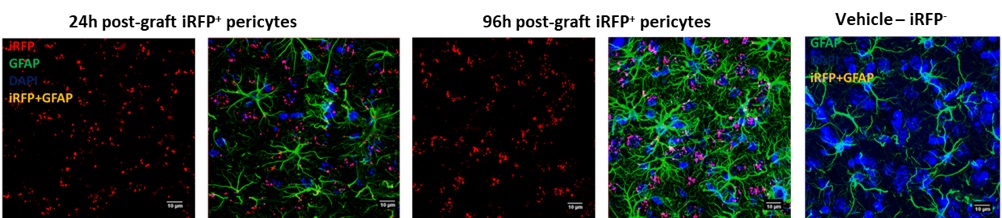
Astrocytes were observed 24h post graft whereas, at 96h post graft the astrocytes were seen highly activated. We also observed colabeling of a few GFAP+ astrocytes with the grafted iRFP+ pericytes (Figure 5). Since astrocytes connect to the basal membrane matrix through cellular end feet adjacent to pericytes and endothelial cells, it is not clearly understood if the grafted pericytes were colabeling with astrocytes or lining the vasculature.
(Text and images by Manju Ganesh, BRNZ PhD student at the University of Otago)